Can Stem Cells Repair Scar Tissue
Abstract
Scars are the normal upshot of wound repair and involve a co-ordinated inflammatory and fibrotic process. When a scar does not resolve, uncontrolled chronic inflammation tin persist and elicits excessive scarring that leads to a range of abnormal phenotypes such as hypertrophic and keloid scars. These pathologies result in significant impairment of quality of life over a long menstruation of time. Existing treatment options are generally unsatisfactory, and at that place is mounting interest in innovative jail cell-based therapies. Despite the interest in mesenchymal stalk cells (MSCs), there is yet to be a human clinical trial that investigates the potential of MSCs in treating abnormal scarring. A synthesis of existing testify of animal studies may therefore provide insight into the barriers to human application. The aim of this PRISMA systematic review was to evaluate the effectiveness of MSC transplantation in the handling of hypertrophic and keloid scars in in vivo models. A total of eleven case-command studies were identified that treated a total of 156 subjects with MSCs or MSC-conditioned media. Ten studies assessed hypertrophic scars, and one looked at keloid scars. All studies evaluated scars in terms of macroscopic and histological appearances and about incorporated immunohistochemistry. The included studies all found improvements in the in a higher place outcomes with MSC or MSC-conditioned media without complications. The studies reviewed support a function for MSC therapy in treating scars that needs further exploration. The transferability of these findings to humans is express by factors such equally the reliability and validity of the disease model, the need to identify the optimal MSC cell source, and the outcome measures employed.
Introduction
Wounds to the skin are caused past mechanical, thermal, and chemical trauma. Scars (or cicatrix) are the normal effect of wound repair and involve a co-ordinated inflammatory and fibrotic process. Eventually, the scars remodel and become soft, flat, pale, and unobtrusive. When a scar does non resolve, persistent chronic inflammation can crusade excessive scarring that pb to a range of aberrant phenotypes which clinically manifest equally hypertrophic and keloid scars.
Hypertrophic scars bear upon nearly one in 5 people who suffer from burns and the hazard of scarring increases with the time taken to heal (Chipp et al. 2022). They tin also occur following incisional closure, a standard part of surgical procedures. Typically appearing inside 2 months of injury, the illness procedure can be protracted and therefore carries significant societal and financial cost over a long period of time (Gangemi et al. 2008). Keloid scars impact tens of millions of people worldwide, and there is strong show of a significant genetic predisposition (Bayat et al. 2003; Santos-Cortez et al. 2022). In dissimilarity to hypertrophic scars, keloid scars tin can appear much subsequently post-injury and are characterised by extension beyond the original expanse of the trauma. Ultimately, hypertrophic and keloid scars result in significant impairment of quality of life (Bock et al. 2006). In addition to corrective consequences, these abnormal scars can take functional implications including restricted mobility, hurting, and pruritus (Bijlard et al. 2022; Lee et al. 2004).
Excess scarring may persist and often recurs after multiple interventions (Darzi et al. 1992; Gauglitz et al. 2022). Near patients suffer from neuropathic pain and pruritus, and the mainstay of handling is bourgeois therapy (Argirova et al. 2006). However, existing handling options are generally unsatisfactory for patients and doctors alike. In particular, surgery, which is mainly focused on scar excision, has a very loftier recurrence rate whether used solitary or in combination with depot steroids (Berman et al. 2009; Furtado et al. 2022; Wilson 2022). Strategies aimed at scar growth suppression include topical treatments such as retinoic acid, imiquimod, and corticosteroid injections (Jacob et al. 2003; Janssen De Limpens 1980). These remedies tend to demonstrate just brusk-term efficacy (Berman et al. 2009; Cação et al. 2009). Repeated steroid injections are notwithstanding efficacious. Pressure therapy and silicone gel foam or sheets stand out equally clinically useful and widely used measures both therapeutically and preventatively (Ai et al. 2022; Kim et al. 2022). Modalities such as radiotherapy, cryotherapy, and lasers take either loftier failure rates, and/ or carry risk of agin events, not to mention high cost (Manuskiatti and Fitzpatrick 2002; Puri and Talwar 2009; Song et al. 2022; Steinstraesser et al. 2022). Therefore, there is mounting interest in innovative methods to treat hypertrophic and keloid scars. Emerging studies have therefore taken a different arroyo and focussed on cell-based therapies such equally mesenchymal stem cells (MSCs) (Fung et al. 2022).
MSCs are developed multipotent stromal cells that can exist readily harvested from various sites such every bit os marrow, adipose, and umbilical tissue (Baksh et al. 2007; Khan et al. 2008). MSCs can be expanded ex vivo and cultured under specific conditions to promote particular cellular effects. Due to their low immunogenicity, MSCs are oft transplanted allogeneically for the treatment of inflammatory conditions (Kabat et al. 2022). MSCs exert their anti-inflammatory and anti-fibrotic paracrine effects via the chemokines and microvesicles that they secrete (Badiavas et al. 2003; Horwitz and Dominici 2008; Rani et al. 2022). Excessive scarring involves undesired inflammation that results in deposition of immature extracellular matrix (ECM) by fibroblasts and myofibroblasts (Barallobre-Barreiro et al. 2022). Whilst tissue native MSCs play a key role in potentiating this process, there is evidence to suggest that transplanted MSCs are instead able to attenuate inflammation and promote a return to homeostasis (Chen et al. 2009; Ren et al. 2008). MSCs may reach this by mediating macrophage course switch from a proinflammatory M1 to anti-inflammatory M2 phenotype (Cho et al. 2022). MSCs also have the potential to negatively modulate ECM deposition, perchance via promoting a T-jail cell response that results in the downregulation of TGF-β1, a central regulator of collagen synthesis (Huang et al. 2022; Spiekman et al. 2022).
Despite the interest in MSCs, at that place is yet to exist a human clinical trial that investigates the potential of MSCs in treating excessive scarring. A synthesis of existing evidence of animate being studies volition therefore provide insight into the barriers to human application. The aim of this systematic review was to evaluate the effectiveness of MSC transplantation in the treatment of hypertrophic and keloid scars in in vivo models.
Materials and methods
A literature search was performed using PubMed, Web of Science, and Cochrane Database from conception to May 2022. The following search terms were used: ((((((((MSC) OR Mesenchymal Stalk Cell) OR Mesenchymal Stromal Prison cell) OR Multipotent Stem Cell) OR Multipotent Stromal Jail cell) OR Stalk Cell)) AND ((Keloid) OR Hypertrophic)) AND Scar.
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and included case control, cohort studies, example series, and randomised controlled trials (Moher et al. 2009). A full of 1098 studies were subjected to the inclusion/exclusion criteria, yielding a final 11 studies for qualitative analysis (Fig. i). Studies that evaluated MSC or MSC-conditioned media transplantation equally therapies were included. Studies that assessed in vivo models were included. Studies of all design were included. Literature reviews, systematic reviews, and case reports were excluded simply were reverse-reference searched to maximise yield. Studies with merely in vitro experiments were excluded. All included studies were published in the English linguistic communication, and all unpublished, inaccessible, and retracted literature were excluded. CB and KT carried out the search independently. Chance of bias was assessed by AH and JS using the SYRCLE RoB tool (Table 1; Fig. 2) (Hooijmans et al. 2022).
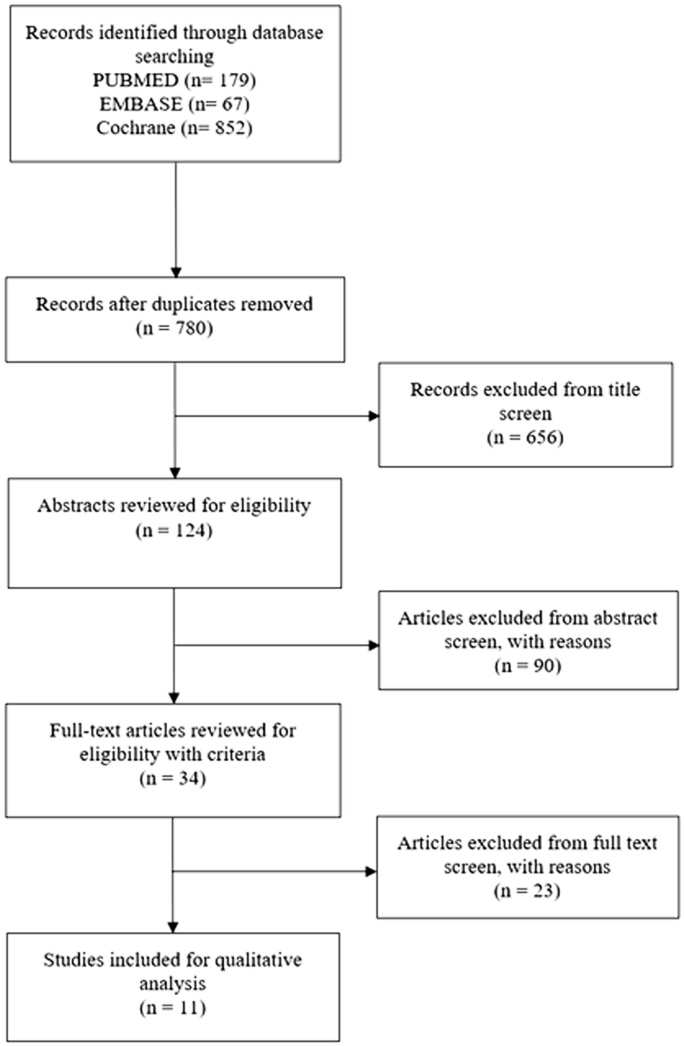
Catamenia diagram of search strategy
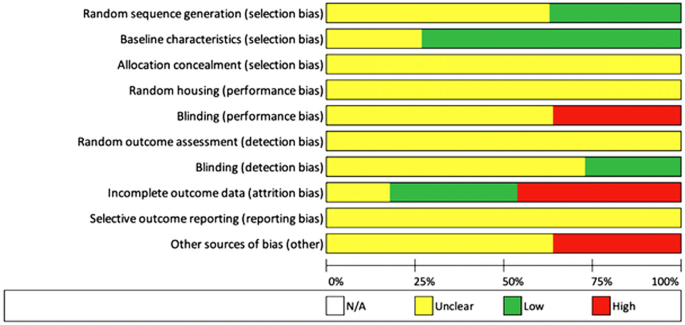
Overall risk of bias
Results
A total of eleven studies were identified (Tables 2, 3, and 4) (Domergue et al. 2022; Foubert et al. 2022; Hu et al. 2022, 2022; Li et al. 2022; Liu et al. 2022, 2022; Yates et al. 2022; Yates et al. 2022; Zhang et al. 2022). A full of 156 subjects were treated with MSCs or MSC-conditioned media. There were no pregnant complications reported in any of the studies. Ten studies assessed the effectiveness of MSCs or MSC-conditioned media in treating hypertrophic scars and one in keloid scars. All studies were example control studies.
MSC isolation and characterisation
Six studies used bone marrow MSCs: two of murine origin (Hu et al. 2022, 2022), two of man origin (Yates et al. 2022; Yates et al. 2022), one of rabbit origin (Liu et al. 2022), and i study included both man and rabbit origin MSCs (Liu et al. 2022). Five of the studies that employed bone marrow MSCs harvested cells by needle aspiration from either the tibia, femur or posterior iliac crest (Hu et al. 2022, 2022; Liu et al. 2022; Liu et al. 2022; Yates et al. 2022). One study, by Yates et al. (Yates et al. 2022) used bone marrow MSCs derived from an immortalised cell line. V studies utilised adipose MSCs: three of human (Domergue et al. 2022; Li et al. 2022; Liu et al. 2022), 1 of porcine (Foubert et al. 2022) and one of rabbit origin (Zhang et al. 2022). Three studies experimented MSCs from inguinal fat pad or redundant tissue from surgical operations (Foubert et al. 2022; Liu et al. 2022; Zhang et al. 2022). Domergue et al. (2016) extracted MSCs by dermolipectomy and Li et al. (2016) past liposuction. Whilst all the studies applied flow cytometry to characterise MSCs, only four satisfied the International Society for Cellular Therapy (ISCT) criteria for defining MSCs by also performing tri-lineage differentiation (Dominici et al. 2006; Hu et al. 2022, 2022; Yates et al. 2022; Yates et al. 2022). Five studies performed bi-lineage differentiation only (Li et al. 2022; Liu et al. 2022, 2022; Liu S. et al. 2022; Zhang et al. 2022).
MSC handling and commitment
Most studies passaged MSCs at least three times. Only two studies used MSCs from earlier passage; Domergue et al. (2016) used passage one, and Foubert et al. (2017) did not passage the cells at all. Interestingly, 2 studies harvested MSCs across the eighth passage (Hu et al. 2022, 2022). Studies transplanted varying concentrations of MSCs but at similar volumes of around 200 μl. Two studies administered 1000 μl (Li et al. 2022; Liu et al. 2022), whilst three studies dispensed less than 100 μl of MSCs (Domergue et al. 2022; Liu. et al. 2022; Yates et al. 2022). Yates et al. (Yates et al. 2022) did not specify the quantity given. The routes of MSC administration were highly variable. Eight of the eleven studies delivered MSCs or MSC-conditioned media by subcutaneous injection. Of these, four studies specified further; two injected iv points of the wound (Domergue et al. 2022; Liu et al. 2022), one injected into the eye of each wound (Zhang et al. 2022), and the fourth delivered MSCs past circumferential intradermal injection into each wound (Liu et al. 2022). Of the remaining three studies, one report delivered MSCs onto the wound via an droplets (Foubert et al. 2022), one applied the MSCs to fill the wound defect (Yates et al. 2022), and i injected MSCs intra-arterially (Liu et al. 2022). Five of the xi studies utilised MSC-conditioned media (Hu et al. 2022, 2022; Li et al. 2022; Liu et al. 2022; Zhang et al. 2022). 2 studies used chemokine receptor 3 (CXCR3) knockout mice, which are known to scar excessively when wounded (Yates et al. 2022; Yates et al. 2022). Four studies employed internal controls by injecting MSCs on the contralateral side of the animal subject (Foubert et al. 2022; Hu et al. 2022; Yates et al. 2022; Zhang et al. 2022). Five studies utilised Dulbecco'due south modified Eagle media (DMEM) (Hu et al. 2022, 2022; Li et al. 2022; Liu et al. 2022; Zhang et al. 2022), and iii applied phosphate buffer solution (PBS) as controls (Domergue et al. 2022; Liu et al. 2022; Liu et al. 2022). Other control groups comprised lactated Ringer'southward solution (LR), hyaluronic acid (HA), and no handling every bit a control (Foubert et al. 2022; Yates et al. 2022; Yates et al. 2022). The majority of studies followed up wound progression for at least 28 days.
Disease model
Viii studies evaluated the effectiveness of MSCs in preventing hypertrophic scar germination (Table 3), and iii studies examined MSC therapy on formed scars. In the latter, one study assessed keloid scars and included four subjects. Six studies assessed murine, iv used rabbit, and 1 utilised a porcine subject. All the induced wounds were total dermal-thickness just varied in size and location, with the majority being round dial wounds inflicted on the dorsum of murine subjects. Four studies inflicted full-thickness punch wounds on the ears of rabbit subjects (Hu et al. 2022; Liu et al. 2022; Liu et al. 2022; Zhang et al. 2022). Three studies (Tabular array 4) created full-thickness skin wounds on homo peel samples which were then xenografted onto murine subjects (Domergue et al. 2022; Hu et al. 2022; Liu et al. 2022).
Treatment outcomes and complications
All studies assessed wounds in terms of macroscopic appearance and histology with about including immunohistochemistry. No complications were reported by any of the studies. Gross appearance was evaluated in all studies using high-resolution photography, and all studies reported positive improvements in various measured parameters in the MSC-treated group compared with controls. Eight studies described reduced scar hypertrophy in the MSC-treated group compared with controls (Domergue et al. 2022; Foubert et al. 2022; Hu et al. 2022, 2022; Li et al. 2022; Yates et al. 2022; Yates et al. 2022; Zhang et al. 2022). Two studies reported that MSC-treated subjects attenuated hypertrophic scar formation (Liu et al. 2022; Liu et al. 2022). One report evaluated keloid size and institute greater scar shrinkage following handling (Liu et al. 2022). Several studies assessed collagen characteristics using assays of collagen gel contraction (Hu et al. 2022), collagen deposition (Foubert et al. 2022; Hu et al. 2022), and collagen content (Domergue et al. 2022). All studies reported reduced collagen deposition and reduced collagen contracture in the MSC-treated group compared with controls. Two studies assessed fibroblast apoptosis. Hu et al. (2020) found increased fibroblast apoptosis by staining for caspase-7. Liu et al. (2018) measured the presence of phosphatidylserine in the outer layer of the phospholipid bilayer as a surrogate marker of apoptosis and found no alter in the MSC-treated group compared with control. Another written report used TUNEL (last deoxynucleotidyl transferase dUTP nick end labelling) staining to assess MSC apoptosis and found that a significant proportion of MSCs underwent apoptosis afterwards administration onto a wound (Liu et al. 2022). 3 of the 11 studies assessed scar thickness, with two using digital planimetry (Foubert et al. 2022; Liu et al. 2022) and one using ultrasonography (Zhang et al. 2022). All studies reported reduced scar tissue height and hardness. Yates et al. (2017), by staining caspase-three with a fluorescent probe, found reduced caspase-3, suggesting improved fibroblast survival following MSC co-transplantation.
Discussion
Although the outcomes reported in this review by and large favour MSC transplantation in treating excessive scarring and did not written report complications, it is difficult to describe reliable conclusions due to the heterogeneity of the studies. This arises from various aspects; there was significant variability in the cell source, cell treatment, method of delivery, and the disease model used to appraise efficacy. Almost studies demonstrated moderate to high overall risk of bias as they were aiming to different and more specific questions relevant to MSC utilize. However, this systematic review provides a useful summary and helps inform futurity study pattern.
The properties of MSCs can vary according to the cell source. Consistent with the existing literature, most of our studies examined adipose MSCs (AMSCs) and os marrow MSCs (BMMSCs) (Kabat et al. 2022). Both of these cell sources have their relative advantages for use in treating scars. AMSCs offer a greater capacity to proliferate ex vivo compared with other jail cell sources (Peng et al. 2008) and therefore may be suitable for large scale off-the-shelf preparations at greater toll-effectiveness. They may also be more abundant, less invasive to harvest, and are frequently bachelor as medical waste in many cosmetic surgery procedures. BMMSCs may represent a less heterogenous cell population (Liu et al. 2022) but exhibit senescence at earlier passage (Couch et al. 2022). An of import consideration is that the anti-inflammatory backdrop of MSCs could differ by cell source. Particular studies suggest that AMSCs may be superior in promoting an M1 to M2 phenotype transition in macrophages that favour resolution of inflammation (Heo et al. 2022). This is relevant equally macrophages are a key mediator of the pathogenic process of excessive scarring (Feng et al. 2022; Hesketh et al. 2022). In addition, sure MSCs demonstrate a greater ability to engraft onto lesions and can therefore produce more sustained effects (Burk et al. 2022). Ane study in this review compared human and rabbit prison cell sources and found both cell sources to be as efficacious (Liu et al. 2022). Harvesting MSCs from animals rather than humans may be more than convenient but the immunogenic consequences of xenogeneic transplantation with human recipients are yet to exist thoroughly investigated. Although heterogeneity of MSC origin and culture status among the included studies may affect the reliability of conclusions drawn from them, it is reassuring that positive furnishings were observed across multiple cell sources. This indicates that MSCs regardless of origin have the potential to treat hypertrophic and keloid scars. Future studies should aim to identify the best prison cell source for treating excessive scarring.
Significant heterogeneity was also observed betwixt the studies in terms of culture weather condition and handling delivery methods. The literature suggests that pre-conditioning MSCs with inflammatory cytokines may serve to promote an anti-inflammatory MSC phenotype (Saldaña et al. 2022). Similarly, following co-civilisation with fibroblasts, a cell type prevalent in inflamed scars, MSCs express greater levels of anti-inflammatory cytokines (Suzuki et al. 2022). This suggests that treating MSCs in conditions cogitating of the scar environs might potentiate their effectiveness when used in transplantation. Conversely, serum-free culture conditions appear to enhance the anti-fibrotic properties of MSCs in vivo (Yoshida et al. 2022). This may represent a potential claiming as the optimal civilisation protocol should promote an anti-fibrotic response without compromising the anti-inflammatory properties of MSCs. I way of circumventing this could be to stimulate MSCs under a particular set of culture conditions, and so harvesting the conditioned media that contains bioactive extracellular vesicles (EVs). The MSCs can then exist resuspended and grown under a different prepare of culture weather to promote secretion of dissimilar bioactive substances. Indeed, several studies in our review showed that a jail cell-free handling using MSC-conditioned media tin can be effective (Hu et al. 2022, 2022; Li et al. 2022; Zhang et al. 2022).
On the other paw, it is difficult to identify the best MSC delivery method. MSCs injected into the apportionment appear to engraft well into wounds (Deng et al. 2005), but carry a risk of interacting with cytokines and drugs nowadays in the serum, which may alter MSC function (Javorkova et al. 2022). In dissimilarity, MSCs injected directly into a lesion of interest could delocalise speedily (Burk et al. 2022) and therefore still have the potential to exert off-site effects (Devine et al. 2003). Although there were no complications reported in any of the studies in this review, several factors have the potential to influence MSC biodistribution and therefore clinical efficacy following administration. It has been reported that pulmonary complications relating to Four administration of MSCs could be dependent on the cell break formulation (Deak et al. 2022). Other studies suggest that following initial localisation in the lungs post-obit systemic administration, MSCs can dwelling house to areas of inflammation (Rustad and Gurtner 2022). Although useful in cases of isolated peel pathology, undesired offsite effects may be observed in cases of other underlying systemic inflammation (Gholamrezanezhad et al. 2022). There is also evidence to testify that the migration and proliferation of MSCs at skin wounds can be a function of MSC expression of adhesion molecules including junction adhesion molecule A (JAM-A) (Wu et al. 2022). Likewise, chemokines such as CCR7 likewise announced to promote MSC migration to skin wounds (Sasaki et al. 2008). For the purposes of treating scars, it appears that local administration may exist preferable, with recent studies demonstrating rubber in animals via subcutaneous (Tappenbeck et al. 2022) and topical (Beyazyildiz et al. 2022) routes. Robust experiments that compare methods of MSC delivery in treating scars should address this ambiguity.
It remains uncertain whether interpretations fatigued from animal models of excessive scarring can be transferred directly to inform treatment in humans. Most of the studies in this review assessed the effects of MSCs on the caste of hypertrophy during the scarring procedure. This probably does not replicate the homo affliction where patients typically present with a fully formed scar. Nevertheless, it may inform whether MSCs can exist implemented at the time of injury (in high risk patients) or shortly after or in conjunction with surgical scar treatment as a means of preventing master or recurrent hypertrophic or keloid scars. Genetic models of hypertrophic scarring may confer high reproducibility. There are existing gain-of-function models such as the Tight Peel two mouse which showroom increased fibrosis following injury (Long et al. 2022), presumably due to increased collagen III alpha-1 expression (Long et al. 2022). Instead of a gain-of-function model, the two studies by Yates et al. (Yates et al. 2022; Yates et al. 2022) captured in this review utilised a previously validated knockdown model by targeting the CXCR3 gene (Yates et al. 2022). Whilst both methods may exist informative for in vivo studies of hypertrophic scarring, they practise not reflect the pattern of genetic predisposition in humans (Zhu et al. 2022), and the knock-down target does non correlate with known protective genetic variants (Sood et al. 2022). It is suggested that concomitantly xenografting human being skin cells into the wound may improve the validity of the mouse burns model by promoting a more extensive scar phenotype (Ibrahim et al. 2022; Momtazi et al. 2022). Nevertheless, this could be confounded by the immunogenic furnishings of xenografting pare onto an immunocompetent mouse (Racki et al. 2022). Nevertheless, the studies in this review that conducted xenografting of human pare into mouse defects did not observe graft rejection (Domergue et al. 2022; Hu et al. 2022; Liu et al. 2022).
Another issue relates to the fourth dimension-course of scar pathogenesis. Most mouse models develop mature hypertrophic or keloid scarring within days to weeks later burn injury and weeks to months after incisional injury (Kim et al. 2022), unlike the longer fourth dimension course of human being illness. In humans, excessive scarring can occur later months (Gangemi et al. 2008), with biomolecular evidence of active disease at up to a yr later (Van Der Veer et al. 2022). There is testify in the literature to support the potential employ of the Cherry-red Duroc porcine model, which develops scarring over months instead, and therefore meliorate recapitulates the human procedure (Harunari et al. 2006; Zhu et al. 2003, 2004). We captured 1 study by Foubert et al. (2017) that was able to utilise this model in guild to undertake an extended follow-upwardly period of six months, when active scar growth was still observed. Whilst all of the studies demonstrated sustained benefit and did non report recurrence up to the finish point of follow-up, keloid and hypertrophic scars are known in humans to recur after many months to years post-obit successful handling (Furtado et al. 2022). Therefore, the short lifespan of murine models may non let sufficient longitude to appraise whether the benefits of MSC therapy is sustained. Future studies of porcine models with long follow-up periods may facilitate this.
In order to fully exploit the beneficial effects of MSCs in treating scars, it is important to establish a dose-response relationship. The studies in this review varied significantly in the amount of MSC or MSC-conditioned media used, only all reported positive outcomes. Only one written report examined the furnishings of varying the dose of MSC-conditioned media used and constitute a dose-response relationship (Li et al. 2022). It is unclear whether the same relationship may be observed in treatment with MSCs of varying concentration and there is evidence in models of ischaemic injury that higher doses of MSCs do non ever confer greater therapeutic benefit (Yavagal et al. 2022). Therefore, a relevant futurity written report might aim to determine the maximum tolerated dose (MTD) for MSCs in treating keloid and hypertrophic scars. The method of delivery might influence this, as appropriate dosage for intravenous injection may be derived from the weight of the subject area, whereas intralesional commitment may require the volume of the scar of interest to be calculated. Digital planimetry, every bit employed by several studies here, may exist a viable method of achieving this (Foubert et al. 2022; Liu et al. 2022). Ascertaining the MTD will besides inform safe dosages that practise not evoke adverse effects (Karussis et al. 2022). Six studies in this review treated scars with MSCs, four studies used conditioned media, and 1 compared the two. There has been an emerging trunk of evidence to support the use of conditioned media, which contains bioactive extracellular vesicles (EVs) that may be the agile therapeutic ingredient of MSCs (Furuta et al. 2022). As a cell-free therapy, it is possible that EVs are less immunogenic and may therefore exist more suitable for big-scale product from allogeneic sources (Monguió-Tortajada et al. 2022).
Outcome measures utilised by in vivo studies tin can limit their transferability to humans. Whilst reduction in scar size and improvement in histological advent may reflect the cosmetic benefits of treatment, it is unclear how it affects scar symptoms. Every bit hurting and pruritis are the main symptoms of hypertrophic and keloid scars (Lee et al. 2004), functional assessments in animals may be crucial earlier undertaking human trials. For example, there are well-validated and quantifiable behavioural measures such as voice that reverberate pain in mice (Kurejova et al. 2022). Assessing the degree of physical activity such as time spent earthworks (Shepherd et al. 2022) could potentially reveal the functional implications of contractures resulting from scars, although this could be dependent on the position of the lesion. Aside from looking to reduce the corporeality of scarring, at that place is a range of symptoms that tin be acquired past excessive scarring, and and so separate studies may be required to evaluate the differential benefits of MSC therapy and to determine a personalised approach co-ordinate to the specific symptom.
Determination
The present review suggests that mesenchymal stem cell (MSC) therapy tin exist an effective method of treating hypertrophic and keloid scars across a range of cell sources and creature models and does not cause significant complications. However, there is inadequate high-level evidence of in-human studies to support clinical efficacy in humans. There are several areas that demand to be addressed before proceeding to human trials. This includes the identification of a reliable, reproducible, and validated animal model, and a standardised method of MSC delivery to allow a dose-response relationship to be established. The similar positive results observed to date with MSCs and MSC-conditioned media are encouraging and should exist explored further past assessing the efficacy of MSC-derived extracellular vesicles, as this volition comport significant implications for cost-effectiveness in treating humans at a population scale.
References
-
Wei AJ , Tao LJ , Duo PS , Li LY , Lin DS , Ming H, Bin P et al (2017) The effectiveness of force per unit area therapy (15–25 MmHg) for hypertrophic burn scars: a systematic review and meta-analysis. Scientific Reports seven
-
Argirova M, Hadjiski O, Victorova A et al (2006) Not-operative treatment of hypertrophic scars and keloids subsequently burns in children. Ann Burns Fire Disasters 19(two):80–87
-
Badiavas EV, Abedi M, Butmarc J, Falanga V, Quesenberry P et al (2003) Participation of bone marrow derived cells in cutaneous wound healing. J Cell Physiol 196(ii):245–250
-
Baksh D, Yao R, Tuan RS et al (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and os marrow. Stem Cells 25(6):1384–1392
-
Barallobre-Barreiro J, Woods E, Bell RE, Easton JA, Hobbs C, Eager M, Baig F, Ross AM, Mallipeddi R, Powell B, Soldin Yard, Mayr M, Shaw TJ et al (2019) Cartilage-similar limerick of keloid scar extracellular matrix suggests fibroblast mis-differentiation in affliction. Matrix Biol Plus 4:100016
-
Bayat A, McGrouther DA, Ferguson MWJ et al (2003) Pare scarring. BMJ 326(7380):88–92
-
Berman B, Harrison-Balestra C, Perez OA, Viera Thou, Villa A, Zell D, Ramirez C et al (2009) Treatment of keloid scars mail-shave excision with imiquimod 5% cream: a prospective, double-blind, placebo-controlled pilot study. J Drugs Dermatol viii(5):455–458
-
Emrullah B, Alpaslan PF, Özlem B, Rümeysa HE, Uʇur A, Necati DM, Aynur A, Figen K, Güngör S, Tuncay D et al (2014) Efficacy of topical mesenchymal stalk prison cell therapy in the handling of experimental dry out eye syndrome model. Stem Cells Int 2022
-
Bijlard E, Kouwenberg CAE, Timman R, Hovius SER, Busschbach JJV, Mureau MAM et al (2017) Burden of keloid affliction: a cross-exclusive health-related quality of life assessment. Acta Dermato-Venereologica 97(two):225–229
-
Bock O, Schmid-Ott G, Malewski P, Mrowietz U et al (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297(ten):433–438
-
Burk J, Berner D, Brehm Westward, Hillmann A, Horstmeier C, Josten C, Paebst F, Rossi M, Schubert Southward, Ahrberg AB et al (2016) Long-term cell tracking following local injection of mesenchymal stromal cells in the equine model of induced tendon disease. Prison cell Transpl 25(12):2199–2211
-
Burrow Grand, Hoyland JA, Richardson SM et al (2017) Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro
-
Cação FM, Tanaka V, Messina MC, Lourenzo De et al (2009) Failure of imiquimod 5% cream to prevent recurrence of surgically excised trunk keloids. Dermatol Surg 35(4):629–633
-
Chen L, Tredget EE, Liu C, Wu Y et al (2009) Assay of allogenicity of mesenchymal stalk cells in engraftment and wound healing in mice. PLoS ONE 4(nine)
-
Chipp E, Charles L, Thomas C, Whiting Thousand, Moiemen North, Wilson Y et al (2017) A prospective study of time to healing and hypertrophic scarring in paediatric burns: every day counts. Burns and Trauma 5
-
Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y et al (2014) Mesenchymal stem cells reciprocally regulate the M1/M2 remainder in mouse os marrow-derived macrophages. Exp Mol Med 46(1):e70–e70
-
Darzi MA, Chowdri NA, Kaul SK, Khan 1000 et al (1992) Evaluation of various methods of treating keloids and hypertrophic scars: a 10-year follow-up written report. Br J Plast Surg 45(five):374–379
-
Deak East, Rüster B, Keller L, Eckert G, Fichtner I, Seifried E, Henschler R et al (2010) Suspension medium influences interaction of mesenchymal stromal cells with endothelium and pulmonary toxicity later on transplantation in mice. Cytotherapy 12(2): 260–64. https://pubmed.ncbi.nlm.nih.gov/19929457/ (Oct 11, 2022)
-
Deng W, Han Q, Liao L, Li C, Ge W, Zhao Z, You lot Southward, Deng H, Murad F, Zhao RCH et al (2005) Engrafted bone marrow-derived Flk-ane+ mesenchymal stalk cells regenerate peel tissue. Tissue Eng 11(ane–ii):110–119
-
Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R et al (2003) Mesenchymal stalk cells distribute to a wide range of tissues post-obit systemic infusion into nonhuman primates. Blood 101(8):2999–3001
-
Domergue Southward, Bony C, Maumus M, Toupet K, Frouin Due east, Rigau V, Vozenin M-C, Magalon 1000, Jorgensen C, Noël D et al (2016) Comparison between stromal vascular fraction and adipose mesenchymal stem cells in remodeling hypertrophic scars ed Alexander V Ljubimov. PLOS ONE eleven(5):e0156161
-
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. the international society for cellular therapy position statement. Cytotherapy 8(4):315–317
-
Feng Y, Sun ZLL, Si YW, Jun JZ, Bin HL, Guo ZD, Yong Y, Shun Y, Ming LY, Feng L, Zhou XJ et al (2019) Direct and indirect roles of macrophages in hypertrophic scar formation. Frontiers in Physiology x(AUG)
-
Foubert P, Zafra D, Liu M, Rajoria R, Gutierrez D, Tenenhaus Yard, Fraser JK et al (2017) Autologous adipose-derived regenerative prison cell therapy modulates development of hypertrophic scarring in a red duroc porcine model. Stem Cell Research and Therapy 8(1)
-
Fung Grand, Yuan Y, Atkins H, Shi Q, Bubela T et al (2017) Responsible translation of stem cell research: an assessment of clinical trial registration and publications. Stalk Cell Rep 8(5):1190–1201
-
Furtado F, Hochman B, Ferreira LM et al (2012) Evaluating keloid recurrence afterwards surgical excision with prospective longitudinal scar cess scales. J Plast, Reconstr Aesthet Surg 65(7):e175–e181
-
Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi One thousand et al (2016) Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem CELLS Transl Med 5(12):1620–1630
-
Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo Thousand, Bollero D, Ganem J, Capocelli R, Cuccuru F, Cassano P, Risso D, Stella M et al (2008) Epidemiology and take a chance factors for pathologic scarring after burn wounds. Arch Facial Plast Surg 10(2):93–102
-
Gauglitz GG, Korting HC, Pavicic T, Ruzicka T, Jeschke MG et al (2011) Hypertrophic scarring and keloids: pathomechanisms and electric current and emerging treatment strategies. Mol Med 17(1–2): 113–25. https://www.ncbi.nlm.nih.gov/pmc/manufactures/PMC3022978/
-
Gholamrezanezhad A, Mirpour S, Bagheri K, Mohamadnejad M, Alimoghaddam Thou, Abdolahzadeh 50, Saghari M, Malekzadeh R et al (2011) In vivo tracking of 111in-oxine labeled mesenchymal stem cells post-obit infusion in patients with advanced cirrhosis. Nucl Med Biol 38(7): 961–67. https://pubmed.ncbi.nlm.nih.gov/21810549/ (October 11, 2022)
-
Harunari Due north, Zhu KQ, Armendariz RT, Deubner H, Muangman P, Carrougher GJ, Isik FF, Gibran NS, Engrav LH et al (2006) Histology of the thick scar on the female, ruby duroc pig: concluding similarities to human hypertrophic scar. Burns 32(6):669–677
-
Heo JS, Choi Y, Kim HO et al (2019) Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stalk Cells Int 2022
-
Hesketh Yard, Sahin KB, West ZE, Murray RZ et al (2017) Macrophage phenotypes regulate scar germination and chronic wound healing. Int J Mol Sci 18(vii)
-
Hooijmans CR, Rovers MM, Vries De, Rob BM, Leenaars Thou, Ritskes-Hoitinga M, Langendam MW et al (2014) SYRCLE'southward risk of bias tool for animal studies. BMC Med Res Methodol xiv(1):43
-
Horwitz EM, Dominici M (2008) How practise mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy 10(8):771–774
-
Hu CH, Tseng YW, Chiou CY, Lan KC, Chou CH, Tai CS, Da Huang H, Hu CW, Liao KH, Chuang SS, Yang JY, Lee OK et al (2019) Bone marrow concentrate-induced mesenchymal stem jail cell conditioned medium facilitates wound healing and prevents hypertrophic scar formation in a rabbit ear model. Stem Cell Res Ther 10(1):275
-
Hu CH, Tseng YW, Lee CW, Chiou CY, Chuang SS, Yang JY, Lee OK et al (2020) Combination of mesenchymal stalk cell-conditioned medium and botulinum toxin type A for treating man hypertrophic scars. J Plast, Reconstr Aesthet Surg 73(3):516–527
-
Huang S, Wu Y, Gao D, Fu X et al (2015) Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar germination in mice. Cytotherapy 17(7): 922–31. http://world wide web.ncbi.nlm.nih.gov/pubmed/25939802
-
Ibrahim MM, Bond J, Bergeron A, Miller KJ, Ehanire T, Quiles C, Lorden ER, Medina MA, Fisher M, Klitzman B, Selim MA, Leong KW, Levinson H et al (2014) A novel immune competent murine hypertrophic scar contracture model: a tool to elucidate disease mechanism and develop new therapies. Wound Repair Regen 22(6):755–764
-
Jacob SE, Berman B, Nassiri M, Vincek V et al (2003) Topical awarding of imiquimod 5% cream to keloids alters expression genes associated with apoptosis. In British Journal of Dermatology, Supplement. 62–65
-
Janssen De Limpens AMP (1980) The local handling of hypertrophic scars and keloids with topical retinoic acid. Br J Dermatol 103(3):319–323
-
Javorkova E, Vackova J, Hajkova K, Hermankova B, Zajicova A, Holan V, Krulova G et al (2018) The effect of clinically relevant doses of immunosuppressive drugs on human being mesenchymal stem cells. Biomed Pharmacother 97:402–411
-
Kabat K, Bobkov I, Kumar South, Grumet Grand et al (2020) Trends in mesenchymal stem prison cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? STEM CELLS Transl Med nine(one):17–27
-
Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S et al (2010) Prophylactic and immunological effects of mesenchymal stalk cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Curvation Neurol 67(10):1187–1194
-
Khan WS, Tew SR, Adesida AB, Hardingham TE et al (2008) Man Infrapatellar fatty pad-derived stem cells express the pericyte mark 3G5 and show enhanced chondrogenesis later on expansion in fibroblast growth gene-two. Arthritis Res Ther 10(iv):R74
-
Kim M, Kim H, Kang HW et al (2018) Comparative evaluations of hypertrophic scar formation in in vivo models. Lasers Surg Med 50(half dozen):661–668
-
Kim SM, Choi JS, Lee JH, Kim YJ, Jun YJ et al (2014) Prevention of postsurgical scars: comparsion of efficacy and convenience between silicone gel sheet and topical silicone gel. J Korean Med Sci 29(Suppl 3):S249–S253
-
Kurejova M, Nattenmüller U, Hildebrandt U, Selvaraj D, Stösser S, Kuner R et al (2010) An improved behavioural assay demonstrates that ultrasound vocalizations constitute a reliable indicator of chronic cancer hurting and neuropathic pain. Mol Pain 6:18
-
Lee SS, Yosipovitch Thousand, Chan YH, Goh CL et al (2004) Pruritus, pain, and small nerve cobweb part in keloids: a controlled written report. J Am Acad Dermatol 51(half dozen):1002–1006
-
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J, Yang X, He T, Guan H, Zheng Z, Han Due south, Dong G, Han J, Shi J, Hu D et al (2016) Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the P38/MAPK signaling pathway. Stem Jail cell Res Ther 7(1):102
-
Liu J, Ren J, Su L, Cheng S, Zhou J, Ye X, Dong Y, Sunday S, Qi F, Liu Z, Pleat J, Zhai H, Zhu N et al (2018) Human adipose tissue-derived stem cells inhibit the activity of keloid fibroblasts and fibrosis in a keloid model by paracrine signaling. Burns 44(ii):370–385
-
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang Y, Yu C, Jin Y et al (2014) Mesenchymal stem cells forbid hypertrophic scar germination via inflammatory regulation when undergoing apoptosis. J Investig Dermatol 134(10):2648–2657
-
Liu Ten, Wang Z, Wang R, Zhao F, Shi P, Jiang Y, Pang X et al (2013) Direct comparison of the potency of homo mesenchymal stem cells derived from amnion tissue, bone marrow and adipose tissue at inducing dermal fibroblast responses to cutaneous wounds. Int J Mol Med 31(ii):407–415
-
Liu YL, Liu WH, Sun J, Hou TJ, Liu YM, Liu Hr, Luo YH, Zhao NN, Tang Y, Deng FM et al (2014) Mesenchymal stalk jail cell-mediated suppression of hypertrophic scarring is P53 dependent in a rabbit ear model. Stem Jail cell Res Ther five(vi)
-
Long KB, Li Z, Burgwin CM, Choe SG, Martyanov V, Sassi-Gaha S, Earl JP, Eutsey RA, Ahmed A, Ehrlich GD, Artlett CM, Whitfield ML, Blankenhorn EP et al (2015) The Tsk2/+ mouse fibrotic phenotype is due to a gain-of-function mutation in the piiinp segment of the Col3a1 gene. J Investig Dermatol 135(3):718–727
-
Long KB, Artlett CM, Blankenhorn EP et al (2014) Tight skin 2 mice exhibit a novel fourth dimension line of events leading to increased extracellular matrix deposition and dermal fibrosis. Matrix Biol 38:91–100
-
Manuskiatti W, Fitzpatrick RE (2002) Treatment response of keloidal and hypertrophic sternotomy scars: comparing among intralesional corticosteroid, v-fluorouracil, and 585-nm flashlamp-pumped pulsed-dye laser treatments. Arch Dermatol 138(9):1149–1155
-
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes Chiliad, Atkins D, Barbour V, Barrowman Due north, Berlin JA, Clark J, Clarke Thou, Cook D, D'Amico R, Deeks JJ, Devereaux PJ, Dickersin K, Egger M, Ernst E et al (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Medicine vi(7)
-
Momtazi M, Kwan P, Ding J, Anderson CC, Honardoust D, Goekjian S, Tredget EE et al (2013) A nude mouse model of hypertrophic scar shows morphologic and histologic characteristics of human hypertrophic scar. Wound Repair Regen 21(i):77–87
-
Monguió-Tortajada M, Roura S, Gálvez-Montón C, Pujal JM, Aran K, Sanjurjo L, Franquesa Thousand, Sarrias MR, Bayes-Genis A, Borràs Fe et al (2017) "Nanosized UCMSC-Derived Extracellular Vesicles but Not Conditioned Medium Exclusively Inhibit the Inflammatory Response of Stimulated T Cells: Implications for Nanomedicine. Theranostics seven(2): 270–84
-
Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C et al (2008) Comparative analysis of mesenchymal stalk cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17(4):761–773
-
Puri Northward, Talwar A (2009) The efficacy of silicone gel for the treatment of hypertrophic scars and keloids. J Cutan Aesthet Sur 2(two):104
-
Racki WJ, Covassin L, Brehm M, Pino Due south, Ignotz R, Dunn R, Laning J, Graves SK, Rossini AA, Shultz LD, Greiner DL et al (2010) NOD-Scid IL2rγnull mouse model of human skin transplantation and allograft rejection. Transplant 89(v):527–536
-
Rani South, Ryan AE, Griffin Doc, Ritter T et al (2015) Mesenchymal stem prison cell-derived extracellular vesicles: toward cell-gratuitous therapeutic applications. Mol Ther 23(five):812–823
-
Ren Thou, Zhang L, Zhao X, Xu One thousand, Zhang Y, Roberts AI, Zhao RC, Shi Y et al (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell ii(2):141–150
-
Rustad KC, Gurtner GC et al (2012) Mesenchymal stalk cells habitation to sites of injury and inflammation. Advances in Wound Intendance 1(4): 147–52. /pmc/articles/PMC3623614/?study=abstruse (Oct 11, 2022)
-
Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N et al (2019) Immunoregulatory potential of mesenchymal stem cells post-obit activation by macrophage-derived soluble factors. Stalk Jail cell Res Ther 10(1)
-
Regie Santos-Cortez, Lyn P, Ying Hu, Fanyue Sunday, Fairouz Benahmed-Miniuk, Jian Tao, Kanaujiya Jitendra K, Samuel Ademola, Solomon Fadiora, Victoria Odesina, Nickerson Deborah A, Bamshad Michael J, Olaitan Peter B, Oluwatosin Odunayo M, Leal Suzanne Thousand, Reichenberger Ernst J et al (2017) Identification of ASAH1 every bit a susceptibility factor for familial keloids. Eur J Hum Gen 25(10):1155–61
-
Sasaki Chiliad, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H et al (2008) Mesenchymal stalk cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin jail cell type. J Immunol 180(4): 2581–87. https://pubmed.ncbi.nlm.nih.gov/18250469/ (October 11, 2022)
-
Shepherd AJ, Cloud ME, Cao YQ, Mohapatra DP et al (2018) Deficits in burrowing behaviors are associated with mouse models of neuropathic simply non inflammatory pain or migraine. Front end Behav Neurosci 12
-
Vocal C, Wu H-G, Chang H, Kim IH, Ha SW et al (2014) Adjuvant unmarried-fraction radiotherapy is safe and effective for intractable keloids. J rad res 55(v):912–916
-
Sood RF, Hocking AM, Muffley LA, Ga M, Honari S, Reiner AP, Gibran NS et al (2015) Genome-wide association study of postburn scarring identifies a novel protective variant. Ann Surg 262(four):563–569
-
Spiekman G, Przybyt E, Plantinga JA, Gibbs S, van Der Lei B, Harmsen MC et al (2014) Adipose tissue-derived stromal cells inhibit TGF-Β1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast Reconstr Surg 134(4):699–712
-
Steinstraesser Fifty, Flak E, Witte B, Ring A, Tilkorn D, Hauser J, Langer Due south, Steinau HU, Al-Benna S et al (2011) Pressure level garment therapy alone and in combination with silicone for the prevention of hypertrophic scarring: randomized controlled trial with intraindividual comparison. Plast Reconstr Surg 128(4):306e–313e
-
Suzuki K, Chosa N, Sawada Due south, Takizawa N, Yaegashi T, Ishisaki A et al (2017) Enhancement of anti-inflammatory and osteogenic abilities of mesenchymal stem cells via prison cell-to-cell adhesion to periodontal ligament-derived fibroblasts. Stalk cells int 2022:3296498
-
Tappenbeck Due north, Schröder HM, Niebergall-Roth East, Hassinger FD, Ulf D, Kathrin K, Korinna Grand, Andreas East, Jasmina F, Natasha Y, Scharffetter-Kochanek, Karin M, George F, Orgill DP, Beck J, Frank MH, Ganss C, Kluth MA et al (2019) In vivo safety profile and biodistribution of gmp-manufactured human pare-derived ABCB5-Positive Mesenchymal Stromal Cells for Use in Clinical Trials. Cytotherapy 21(5): 546–threescore. /pmc/articles/PMC6513723/?written report=abstract (October xi, 2022)
-
Der Veer Five, Willem 1000, Niessen FB, Ferreira JA, Zwiers PJ, Jong De, Etty H, Middelkoop Eastward, Molema Grand et al (2011) Fourth dimension course of the angiogenic response during normotrophic and hypertrophic scar germination in humans. Wound Repair Regen 19(iii):292–301
-
Wilson AM (2013) Eradication of keloids: surgical excision followed by a single injection of intralesional 5-fluorouracil and botulinum toxin. Can J Plast Surg 21(2):87–91
-
Wu M, Ji Due south, Xiao S, Kong Z, Fang H, Zhang Y, Ji K, Zheng Y, Liu H, Xia Z et al (2015) JAM-A promotes wound healing by enhancing both homing and secretory activities of mesenchymal stem cells. Clin Sci 129(seven): 575–88. https://pubmed.ncbi.nlm.nih.gov/25994236/ (Oct 11, 2022)
-
Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A et al (2010) Lack of CXC chemokine receptor 3 signaling leads to hypertrophic and hypercellular scarring. Am J Pathol 176(iv):1743–1755
-
Yates CC, Nuschke A, Rodrigues Grand, Whaley D, Dechant JJ, Taylor DP, Wells A et al (2017) Improved transplanted stem prison cell survival in a polymer gel supplemented with tenascin c accelerates healing and reduces scarring of murine skin wounds. Jail cell Transplant 26(1):103–113
-
Yates CC, Rodrigues M, Nuschke A, Johnson ZI, Whaley D, Stolz D, Newsome J, Wells A et al (2017) Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res Ther viii(1)
-
Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, Rangel EB, McNiece I, Rundek T, Sacco RL, Perez-Pinzon M, Hare JM et al (2014) Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS ONE 9(5)
-
Yoshida Ken, Nakashima Ayumu, Doi Shigehiro, Ueno Toshinori, Okubo Tomoe, Kawano Ki, ichiro Kanawa Masami, Yukio Kato, Yukihito Higashi, Takao Masaki et al (2018) Serum-Free Medium Enhances the Immunosuppressive and Antifibrotic Abilities of Mesenchymal Stem Cells Utilized in Experimental Renal Fibrosis. Stem Cells Transl Med 7(12):893–905
-
Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG et al (2015) Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther six(1)
-
Zhu F, Wu B, Li P, Wang J, Tang H, Liu Ye, Zuo X, Cheng H, Ding Y, Wang W, Zhai Y, Qian F, Wang W, Yuan X, Wang J, Ha West, Hou J, Zhou F, Wang Y et al (2013) Clan study confirmed susceptibility loci with keloid in the Chinese Han population. PLoS ONE viii(5):e62377
-
Zhu KQ, Engrav LH, Tamura RN, Cole JA, Muangman P, Carrougher GJ, Gibran NS et al (2004) Further similarities betwixt cutaneous scarring in the female, reddish duroc hog and man hypertrophic scarring. Burns 30(half-dozen):518–530
-
Zhu KQ, Engrav LH, Gibran NS, Cole JK, Matsumura H, Piepkorn M, Isik FF, Carrougher GJ, Muangman PM, Yunusov MY, Yang T-Thou et al (2003) The female, red duroc squealer every bit an creature model of hypertrophic scarring and the potential role of the cones of skin. Burns 29(7):649–664
Writer information
Affiliations
Contributions
CB and KT contributed equally equally first author. The study was designed and supervised by KT, WK, and CMM. JS and AH carried out study quality assessment. KTS conducted qualitative information synthesis. All authors contributed significantly and were involved in editing, reviewing, and blessing the manuscript.
Respective author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The commodity does not contain any studies with man participants or animals performed by any of the authors.
Boosted data
Publisher'southward Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Eatables Attribution iv.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, equally long equally y'all give appropriate credit to the original author(south) and the source, provide a link to the Creative Commons licence, and betoken if changes were made. The images or other third party material in this article are included in the commodity's Creative Commons licence, unless indicated otherwise in a credit line to the cloth. If material is non included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this commodity
Cite this commodity
Bojanic, C., To, K., Hatoum, A. et al. Mesenchymal stem cell therapy in hypertrophic and keloid scars. Cell Tissue Res 383, 915–930 (2021). https://doi.org/10.1007/s00441-020-03361-z
-
Received:
-
Accepted:
-
Published:
-
Issue Engagement:
-
DOI : https://doi.org/10.1007/s00441-020-03361-z
Keywords
- Mesenchymal stem cells
- Scar
- Pain
- Wound healing
- Wound regeneration
Source: https://link.springer.com/article/10.1007/s00441-020-03361-z
Posted by: eckerttoop1970.blogspot.com


0 Response to "Can Stem Cells Repair Scar Tissue"
Post a Comment